Background: High Grade B cell Lymphoma (HGBL) with MYC and BCL2 and/or BCL6 rearrangements(R) was introduced as an entity in 2016 by the WHO revised 4 th edition. In 2022, both the WHO 5 th edition (beta version) and the International Consensus Classification (ICC) separated DHL- BCL2 (+/- BCL6-R)from DHL- BCL6 given differences in biology . However, while the ICC has maintainedDHL- BCL6 as a provisional entity, the WHO has removed the category, thus removing the requirement to FISH for BCL6-R in this setting. Clinical data on DHL- BCL6 is much more limited, as these cases represent only 10-20% of DHL and have been combined with BCL2-R cases in prior studies. Outcomes are variable in retrospective studies with no consistent data on prognosis or optimal therapeutic strategies. By retaining the category of DHL-BCL6 as a provisional entity, the ICC emphasized the need for further, multicenter, prospective studies evaluating the clinical and biological features of this disease. We herein report a comprehensive comparison of clinical characteristics and outcomes in patients with DHL- BCL6 compared to diffuse large B-cell lymphoma (DLBCL), not otherwise specified (NOS); DLBCL with MYC rearrangement only; DHL- BCL2; and HGBL, NOS in a large, multicenter, prospective cohort of patients from Lymphoma Epidemiology of Outcomes (LEO).
Methods: Adult patients with newly diagnosed large B-cell or HGBL were enrolled within 6 months of diagnosis at one the 8 LEO cohort academic medical centers in the US between 2015 and 2020. Baseline characteristics were abstracted at the time of diagnosis per protocol. Based on FISH data patients were further sub mgrouped into DLBCL, NOS (without MYC rearrangement); MYC-R DLBCL, NOS; HGBL, NOS; DHL- BCL2 and DHL- BCL6. Event-free survival (EFS) was defined as the time from diagnosis until progression/relapse, retreatment, or death. Overall survival (OS) was defined as the time from diagnosis until death due to any cause.
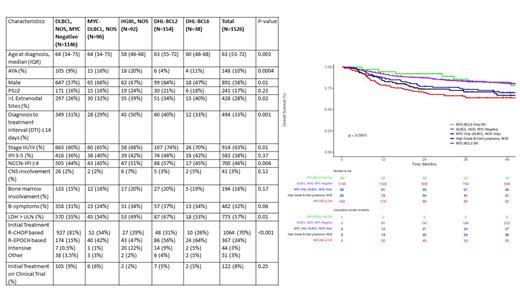
Results: A total of 1526 eligible patients were identified during this time period. All FISH data was available at the time of diagnosis and the choice of treatment was based on physician discretion. Median age at diagnosis was 63 years (IQR 53-72), with 148 (10%) patients in the AYA category, and 128 (8%) patients > 80 years. 58% (891) were male, 11% self identified as Hispanic or Latino, and 7% as Black/African American. The median diagnosis to treatment interval (DTI) was 20 days (IQR 12-32), and 33% had DTI < 14 days. The FISH-based subgroups were MYC-negative DLBCL, NOS (N=1146, 75%), MYC-R DLBCL,NOS 227 (N = 96, 6%), DHL- BCL2 (N=154, 10%), DHL- BCL6 (N=38, 3%), and HGBL, NOS (N=92, 6%). When available, COO by Hans algorithm was 92% GCB in DHL- BCL2 and 50% GCB in DHL- BCL6.
Clinical characteristics can be found in the table. At a median follow-up of 49 months (IQR 36-67), 490 patients (32%) had an event and 356 patients (23%) died. EFS at 24 months (EFS24) was 75% (95% CI: 73-77). Patients with DHL- BCL6 were younger at diagnosis (median 60 years), had more extranodal site involvement (40%), more often stage III/IV disease (70%), and more often treated with a higher intensity regimen than R-CHOP (69%) compared to DLBCL,NOS and MYC-R DLBCL. DHL- BCL6 also had fewer patients that were males (47%), with DTI <=14 days (33%), NCCN IPI ≥ 4 (45%), elevated LDH (53%) than HGBL, NOS and DHL- BCL2. The 2-year EFS and OS rates were noted to be significantly better in the DHL- BCL6 (EFS 79%, 95% CI: 67-93; OS 92%, 95% CI: 84-100) as compared to DHL- BCL2 (EFS 58%, 95% CI: 50-66; OS 70%, 95% CI 63 - 78) and HGBL, NOS (EFS 74%, 95% CI: 65-84; OS 74%, 95% CI: 65-84 ), (Figure 1) but were comparable to that of DLBCL, NOS (EFS 78%, 95% CI: 76-81; OS 87%, 95% CI: 86-89).
Conclusions: Our data support separating DHL- BCL6 from DHL -BCL2 as these patients form a unique subgroup with some clinical characteristics comparable to both DLBCL, NOS as well as HGBL, NOS and DHL- BCL2 subtypes. In this cohort, clinical outcomes are more comparable to DLBCL, NOS than DHL- BCL2 or HGBCL, NOS. More frequent use of intensive chemotherapy in DHL- BCL6 compared with DLBCL may account for this finding, although larger multicenter studies are needed. Our results support continued identification of DHL- BCL6 in the clinical setting to better understand optimal therapy and biology of this cohort.
Disclosures
Cerhan:Protagonist: Other: Safety Monitoring Committee; NanoString: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Research Funding; Genentech: Research Funding. Cohen:Novartis: Research Funding; BMS/Celgene: Research Funding; Genentech: Research Funding; BioInvent: Research Funding; Lam Therapeutics: Research Funding; Takeda,: Research Funding; ADCT: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy; Janssen: Consultancy; BeiGene: Consultancy; Loxo/Lilly: Consultancy, Research Funding. Flowers:Cellectis: Research Funding; Allogene: Research Funding; Amgen: Research Funding; Guardant: Research Funding; Xencor: Research Funding; Sanofi: Research Funding; Pharmacyclics: Research Funding; Iovance: Research Funding; Adaptimmune: Research Funding; Acerta: Research Funding; TG Therapeutics: Research Funding; Takeda: Research Funding; 4D: Research Funding; Spectrum: Consultancy; SeaGen: Consultancy; Pharmacyclics Jansen: Consultancy; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Karyopharm: Consultancy; Gilead: Consultancy, Research Funding; Genmab: Consultancy; Genentech Roche: Consultancy, Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Kite: Research Funding; Morphosys: Research Funding; Nektar: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Ziopharm: Research Funding; Burroghs Wellcome Fund: Research Funding; V Foundation: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; Jannsen Pharmaceuticals: Research Funding; Denovo Biopharma: Consultancy; Celgene: Consultancy, Research Funding; Beigene: Consultancy; Abbvie: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; Eastern Cooperative Oncology Group: Research Funding; National Cancer Institute: Research Funding; CPRIT Scholar in Cancer Research: Research Funding. Lossos:BeiGene: Consultancy; NCI: Research Funding; University of Miami: Current Employment; NCI: Research Funding; Adaptive: Honoraria; LRF: Membership on an entity's Board of Directors or advisory committees. Nastoupil:Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; Gilead Sciences/Kite Pharma: Honoraria, Research Funding; AstraZeneca: Honoraria; Regeneron: Honoraria; Daiichi Sankyo: Honoraria, Research Funding; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; ADC Therapeutics: Honoraria; AbbVie: Honoraria. Martin:AbbVie, AstraZeneca, Beigene, Epizyme, Genentech, Gilead, Janssen, Pepromene, Daiichi Sankyo: Consultancy. Nowakowski:Bantam Pharmaceutical LLC: Consultancy; TG Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Blueprint Medicines: Consultancy; Genentech: Consultancy; Ryvu Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Debiopharm: Consultancy; F Hoffmann-La Roche Limited: Consultancy; Fate Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene Corporation: Consultancy; Selvita Inc: Consultancy; Seagen: Consultancy; MEI Pharma: Consultancy; Kymera Therapeutics: Consultancy; Kite Pharma: Consultancy; Incyte: Consultancy; Karyopharm Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy; Curis: Consultancy; Zai Lab Limited: Consultancy. Maurer:AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Research Funding; Roche/Genentech: Research Funding. Habermann:sorrento: Research Funding; Genentech: Research Funding; BMS: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal